Researchers have made significant strides in understanding the carbonation process in cement-based materials, which could play a crucial role in reducing atmospheric carbon dioxide (CO2).
Carbonation, a process where CO2 reacts with cement compounds to form stable minerals, has been studied extensively for its potential to capture and store CO2. However, the exact mechanisms driving this process remain unclear due to the complex and unstable nature of the materials involved.
In a recent study, published in The Journal of Physical Chemistry C, a team of researchers led by Associate Professor Takahiro Ohkubo from Chiba University, along with collaborators from The University of Tokyo, University of Ryukyus, Hiroshima University, and Hokkaido University, investigated the carbonation process in greater depth.
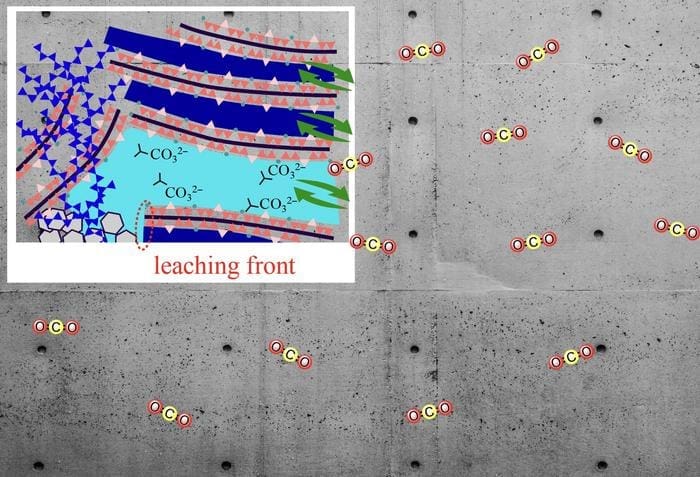
“The role of water transport and carbonation-related structural changes remains an open question. In this study, we used a new method to study these factors, using 29Si nuclear magnetic resonance (NMR) and 1H NMR relaxometry, which has been established as an ideal tool for studying water transport in C–S–H (n.r. calcium silicate hydrates),” says Associate Professor Ohkubo.
To overcome the challenges of studying natural carbonation, which occurs over decades, the researchers used accelerated carbonation experiments, exposing synthesized C–S–H samples to 100% CO2 – far higher than atmospheric levels.
“Natural carbonation in cement materials occurs over several decades by absorbing atmospheric CO2, making it difficult to study in a lab setting. Accelerated carbonation experiments with elevated CO2 concentrations provide a practical solution to this challenge,” explains Ohkubo.
The experiments were conducted under varying RH conditions and Ca/Si ratios, and the team used 29Si NMR to study structural changes, along with 1H NMR relaxometry to track water transport under a deuterium dioxide (D2O) atmosphere.
The study revealed that carbonation-induced structural changes, including the collapse of the C–S–H chain and alterations in pore size, were significantly influenced by both the Ca/Si ratio and RH conditions. Lower RH levels and a high Ca/Si ratio resulted in smaller pores, which suppressed the movement of Ca2+ ions and water, leading to inefficient carbonation.
“Our study shows that the carbonation process occurs due to a combination of structural modifications and mass transfer, signifying the importance of studying their interplay, rather than just structural changes,” adds Ohkubo.
The findings could have far-reaching implications, according to the researchers. “Our findings can contribute to developing new building materials that can absorb large amounts of atmospheric CO2. Additionally, carbonation reactions are also common in organic matter, and hence, our new approach will also help to understand the carbonation of compounds in the natural environment,” says Associate Professor Ohkubo.
This research marks a significant step forward in the quest to reduce CO2 emissions through advanced building materials capable of absorbing atmospheric CO2. Understanding the intricacies of the carbonation process could open new doors to developing more efficient materials for carbon capture and storage.
***
Takahiro Ohkubo is currently an Associate Professor at the Graduate School of Engineering at Chiba University, Japan since 2015. He received his M.S. degree from Chiba University in 2000 and his Ph.D. degree from Tokyo Institute of Technology in 2005. Before joining Chiba University, he worked at the Japan Atomic Energy Agency and the National Institute of Advanced Industrial Science and Technology. His research interests include inorganic chemistry, computational science, and nuclear magnetic resonance. He has published around 60 research articles, which have been cited over 500 times.
Journal Reference:
Taiki Uno, Naohiko Saeki, Ippei Maruyama, Yuya Suda, Atsushi Teramoto, Ryoma Kitagaki, and Takahiro Ohkubo, ‘Understanding the Carbonation Phenomenon of C–S–H through Layer Structure Changes and Water Exchange’, The Journal of Physical Chemistry C 128 (28), 11802-11816 (2024). DOI: 10.1021/acs.jpcc.4c01714
Article Source:
Press Release/Material by Chiba University
Featured image credit: Gerd Altmann | Pixabay