Explore the latest insights from top science journals in the Muser Press daily roundup (November 3, 2025), featuring impactful research on climate change challenges.
In brief:
Extra iron helps stressed out wheat grow up big and strong
Experiments show reducing iron deficiency with a synthetic organic molecule called PDMA results in better growth and healthier plants. These findings are good news for farmers and consumers alike and could lead to treatments in the field that improve wheat production during prolonged periods of heat.
The study was published in the scientific journal Nature Communications.
One of the biggest fears of ongoing climate change is that extended periods of heat will disrupt food production. Even moderate warming can reduce the yield of cool-season cereal crops such as wheat, with one global study estimating that wheat production declines by 6% for every increase of one degree Celsius. Not only do these crops do less photosynthesis and produce smaller kernels of grain, but the grain is also less nutritious.
While most research into how plants adapt to heat stress has focused on acute stress – very high temperatures over a few days – Mochida and his team reasoned that the bigger threat posed by climate change is extended periods of moderately high temperatures.

The researchers characterized what happens to bread wheat after two weeks of moderate heat stress. Compared to wheat grown at normal temperatures, the stressed out wheat plants weighed less and analysis indicated they were doing less photosynthesis. When checking for nutrient deficiencies, the researchers discovered that the leaves from the heat-stressed plants contained less than half the normal amount of iron. Could their stunted growth be the result of iron deficiency?
Genetically, wheat is complex. To get down to the biological details, the researchers turned to a genetically simpler grass called purple false brome (Brachypodium distachyon), which is often used as a model plant for studies of cereal crops. Typically, model plants for experiments come from biobanks that store specific specimens that can then be used by researchers around the world, ensuring consistent genetics every time. For a widely used model like B. distachyon, biobanks contain numerous individual samples, each with a code name and its own slightly different genetics, just like people.
In experiments, the model grass responded to heat stress in almost the same way the wheat had. But the degree to which the grass was affected varied sample by sample, as did the iron deficiency. For example, grass sample Bd21 had extremely low biomass, very yellow leaves, and 91% less iron than plants grown in normal temperatures. On the other hand, sample Bd21-3 had somewhat milder symptoms and only 61% iron deficiency. With the simpler genome, the researchers were able to compare these two model grass samples and pinpoint BdTOM1, the gene responsible for the difference.
Plants cannot extract iron from the soil as is. Instead, they make organic compounds called mugineic acids and dump them in the soil. Once these compounds bind to iron in the soil, the plants can then take it up through their roots. The gene BdTOM1 is responsible for making the mugineic acids. Analysis showed that after two weeks of heat stress, grass sample Bd21-3 had a lot more deoxymugineic acid in its roots than did Bd21, explaining why Bd21 had greater iron deficiency and indicating that variations in BdTOM1 likely lead to variations is heat-stress susceptibility.

The researchers then reasoned that they could alleviate iron deficiency and improve growth by giving heat-sensitive plants more deoxymugineic acid. They tested this hypothesis in the model grass and in wheat using a synthetic deoxymugineic acid called PDMA. Their hypothesis was correct; under heat stress, PDMA treatment led to improved photosynthesis and biomass, provided the PDMA concentration wasn’t too high.
Mochida is optimistic about seeing these findings tested in the field. “In the short term,” he says, “this research proposes a new approach to enhancing crop heat stress tolerance, demonstrating the potential to optimize iron uptake and improve agricultural productivity.”
“In the long term, breeding efforts targeting genes involved in nutrient homeostasis could contribute to food security and sustainable agriculture, considering climate scenarios, societal needs, and resource competition across sectors such as agriculture and energy.”
Journal Reference:
Minami, A., Onda, Y., Shimizu, M. et al., ‘Chelation-based iron uptake mitigates the effects of prolonged high-temperature stress in cool-season grasses’, Nature Communications 16, 7709 (2025). DOI: 10.1038/s41467-025-63005-0
Article Source:
Press Release/Material by RIKEN
Abandoned coal mine drainage could be a significant source of carbon emissions
For the past 250 years, people have mined coal industrially in Pennsylvania, USA. By 1830, the city of Pittsburgh was using more than 400 tons of the fossil fuel every day. Burning all that coal has contributed to climate change. Additionally, unremediated mines – especially those that operated before Congress passed regulations in 1977 – have leaked environmentally harmful mine drainage. But that might not be the end of their legacy.
In research presented at GSA Connects 2025 in San Antonio, Texas, USA, Dr. Dorothy Vesper, a geochemist at West Virginia University, found that those abandoned mines pose another risk: continuous CO₂ emissions from water that leaks out even decades or centuries after mining stops.
In a 2016 study, Vesper and her collaborators found that the water draining from just 140 of these mines in Pennsylvania add as much CO₂ to the atmosphere each year as a small coal-fired power plant. Considering officials and scientists don’t know the number of abandoned mines in Pennsylvania, let alone elsewhere, the impact of these mines is an unresolved and important part of understanding the sources of human-caused climate change.

“We would like to have a much better handle on how big” these carbon emissions are, says Vesper. “A huge part of it is just not even knowing where the discharges are. And it’s not just Appalachia. It’s all over the country. It’s all over the world, really, these mine waters.”
The mine waters, loaded with sulfuric acid as a byproduct of the coal’s geology, break down carbonate rocks like limestone that is associated with the coal seams. Part of that rock is ancient CO₂ that was locked away when the rock formed millions of years ago. The acidic water dissolves the carbonate rocks, releasing carbonate ions (CO₃) that then convert to CO₂ or other forms of carbon in the water. Once the discharge leaves the mine and is exposed to air, any CO₂ present in the water can “degas” and be released into the atmosphere.
Prior to Vesper’s work, CO₂ emissions from degassing of mine drainage hadn’t been extensively quantified. Part of the problem is the sheer number of abandoned mines and the fact that they aren’t well catalogued. Often, Vesper and her students would tromp through the woods to measure a reported mine and find no trace of the opening or that the discharge no longer flowed.
Another problem is that standard field instruments can’t measure extremely high concentrations of CO₂, and Vesper has found that some mine drainage contains up to 1,000 times more CO₂ than would be expected in normal water. To make her measurements, then, Vesper had to turn to an unexpected source for a measuring device.
“It’s basically out of the soda industry. Bottling plants and breweries have them,” says Vesper. The beverage instrument is “designed to be carried around the brewery floor and connect to these giant vats. So, it’s really portable, and it can handle really high CO₂.”
Specialized instrument in hand, Vesper, along with her students and collaborators, track down old mines to measure the CO₂ being carried out through water drainage. The results from some mines were comparable to the CO₂ given off by hydrothermal springs, and vastly higher than water draining from typical natural limestone caves. Further, the amount of CO₂ at each site changed through time dependent on hydrologic conditions around the mine.
In the future, Vesper hopes to measure more mines for longer periods and in different conditions, add methane to her suite of analyses, and explore how different remediation techniques might prevent the CO₂ from being released to the atmosphere and contributing to climate change.
“I think that even just small things in remediation design could make a difference, like keeping the discharge underground in pipes and introducing it to treatment wetlands from the subsurface,” says Vesper. “Then you’re fine. It’s not going to degas in the environment as easily.”
Journal Reference:
Vesper, Dorothy J., Herman, Ellen K., ‘CO₂ Releases from Abandoned Coal Mines in Pennsylvania and West Virginia’, Geological Society of America Abstracts with Program 57 (6) (2025). DOI: 10.1130/abs/2025AM-10087
Article Source:
Press Release/Material by Rudy Molinek | Geological Society of America (GSA)
Expansion microscopy helps chart the planktonic universe
Plankton are the invisible engines of life on Earth, producing much of the planet’s oxygen and forming the foundation of the oceanic food chain. They are also incredibly diverse, with tens of thousands of species described so far, and many more waiting to be discovered. Among them, protists, tiny, single-celled organisms, stand out for their extraordinary diversity and evolutionary significance, yet for decades, scientists could study them only through genomic data, as reliable imaging methods were lacking.
During the COVID-19 pandemic, EMBL Group Leader Gautam Dey received a Zoom call from his collaborator Omaya Dudin, then leading a group at EPFL. Dudin had just adapted a new technique to visualise the internal architecture of Ichthyosporea – a marine protist closely related to animals and fungi – overcoming the long-standing barrier of its impermeable cell walls. The new technique called expansion microscopy, first developed by scientists at MIT, USA, and further optimised into ultrastructure expansion microscopy (U-ExM) for exploring sub-cellular ultrastructure by Paul Guichard and Virginie Hamel at the University of Geneva, had made the cell wall permeable, and the protist’s inner structures could now be clearly visualised and studied.
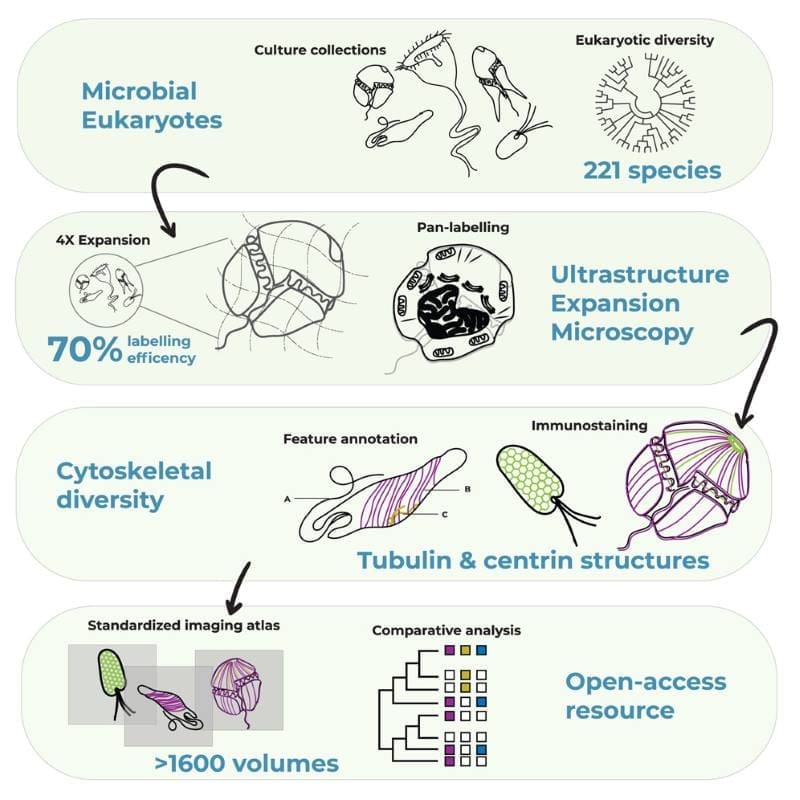
Determined to study more marine organisms with this method, Dudin, Dey, Guichard and Hamel started a cooperation that, three years later, has succeeded in generating almost encyclopedic knowledge of hundreds of protist species and is on its path to creating a planetary atlas of plankton.
The EMBL-led Traversing European Coastlines (TREC) expedition was a great opportunity for the researchers to dive deeper into the internal structures of different marine microbes. Recently published in Cell, their collaboration has yielded unprecedented and in-depth insights into the cellular architecture of over 200 plankton species, in particular eukaryotes – organisms that have a cell nucleus enclosed by a membrane. This was the first step in PlanExM, a TREC plug-in project which aims to reveal the world of planktonic ultrastructural diversity with expansion microscopy.
Unveiling cellular secrets with ultrastructure expansion microscopy
At Roscoff, France, one of the TREC expedition’s first prominent sampling stops, the Station Biologique hosts one of the most complete culture collections of marine microorganisms in Europe. The team asked Ian Probert, the facility’s manager, how many samples they might receive to run an expansion microscopy pilot – expecting about 20 species – only to find the doors of the full facility, with over 200 species, being opened to them.
“We spent three days and nights just fixing those samples. This was a treasure trove we could not let go of,” said co-first author Felix Mikus, who completed his PhD from the Dey Group and is now a postdoc in Dudin’s current lab at the University of Geneva.
Expansion microscopy, a relatively young technique only turning 10 years old this year, works by physically ‘expanding’ biological samples. The sample – which can contain single-celled organisms, cells, or tissues – is first embedded in a clear gel. Then, the gel is allowed to expand by absorbing water. Marvellously, many of the cell’s internal structures remain intact during the process and expand more or less proportionally, allowing scientists to ‘magnify’ the sample four or even 16 times without needing to resort to lenses.
“When combined with regular light microscopy methods, expansion microscopy allows scientists to bypass the standard wavelength barriers which limit how small a structure can be resolved using light microscopy,” said Guichard and Hamel.
Using the Roscoff samples, as well as a second culture collection from Bilbao, Spain, the researchers went on to perform one of the most extensive investigations to date of diversity of the cytoskeleton – the filamentous network that forms the underlying structure of eukaryotic cells. In particular, researchers looked at microtubules – hollow, tubular filaments that help the cell maintain its structure, divide, and move, and centrins – a family of proteins found in the structures that organise microtubules inside cells.
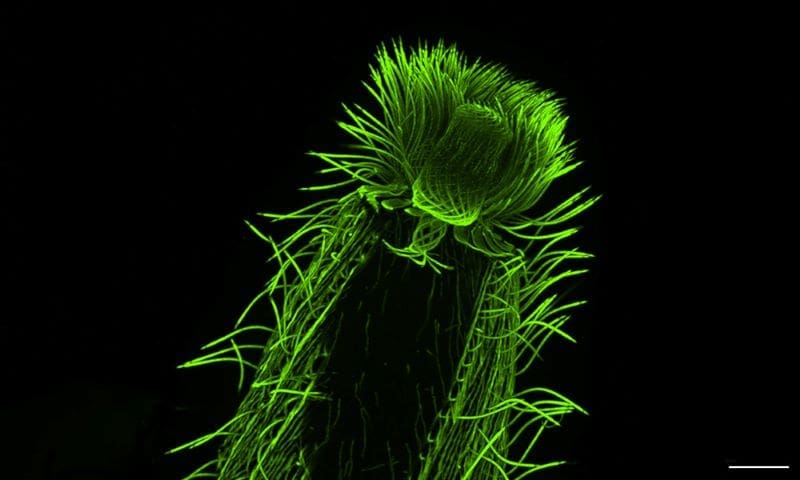
“We were able to map features of microtubule and centrin organisation across many different eukaryotic groups,” said Hiral Shah, EIPOD Postdoctoral Fellow in EMBL’s Dey and Schwab groups and co-first author of the study. “The scale of the study, with many species characterised in each group, opens up the possibility to make evolutionary predictions. For instance, dinoflagellates, one of the most diverse groups found in oceans across the planet, are well-represented in our study. We were able to map the presence of tubulin and centrin structures associated with the cell cortex or the flagella in these species.”
“U-ExM is transforming how we explore protist ultrastructure,” said Armando Rubio Ramos, co-first author of the study and Postdoctoral Fellow at Hamel & Guichard’s research group at the University of Geneva. “By combining this technique with high-throughput imaging and comparative analyses, we can begin to decode how cellular architecture has diversified across evolution. It’s a bridge between molecular data and the physical organisation of life at the microscopic scale.”
According to the researchers, this analysis not only helps us understand the fundamental principles that underlie the organisation of a eukaryotic cell, it also offers clues to the evolutionary history of the cytoskeleton architecture in these species. Moreover, it demonstrates the potential of expansion microscopy as a powerful tool for studying complex samples, including those collected directly from marine ecosystems.
“Our adventures with expansion microscopy are only beginning,” said Dey. “This is perhaps the first high-resolution microscopy technique that has the potential to match the scale and ambition of large biodiversity genomics projects, enabling us in the near future to associate new multiomics data with cellular physiology at scale across the tree of life.”
With Thomas Richards from Oxford University joining the team, Dey and Dudin were also successful in obtaining a prestigious Moore Foundation Grant worth CHF 2 million (about USD 2,500,000) to continue this project.
“The next step is to selectively look deeper into certain species within this broad collection to answer specific questions, such as understanding how mitosis and multicellularity evolved and the phenotypic diversity that underlie major evolutionary transitions,” Dudin said.
Journal Reference:
Mikus, Felix et al., ‘Charting the landscape of cytoskeletal diversity in microbial eukaryotes’, Cell (2025). DOI: 10.1016/j.cell.2025.09.027. Also available on ScienceDirect.
Article Source:
Press Release/Material by Shreya Ghosh | European Molecular Biology Laboratory (EMBL)
Featured image credit: Gerd Altmann | Pixabay




